Lecture 8
Composition space and mineral
stability
Compositions
space
We have
already discussed different ways of writing mineral formulas. There are often times that we want to
express these formulas graphically.
We call this graphic depiction the mineralÕs composition space, the minimum number of chemical
species necessary to describe the composition of mineral phases being
considered. We will see how to use
this approach to (1) plot specific mineral compositions, (2) show, graphically,
the extent of solid solution, and (3) depict chemical reactions among different
mineral phases. In doing this we
will be plotting in two dimensions (because itÕs easiest) É to show more than
two different chemical species, weÕll introduce plotting on ternary diagrams (something
that petrologists like to do!).
Two-component
systems
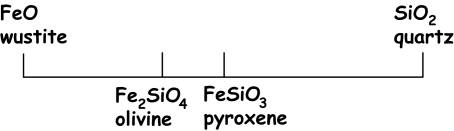
The diagram
above shows the composition space defined by the two-component chemical system
FeO Š SiO2. Note that
the components can be oxide complexes rather than simple ions. In this simple example, the composition
space is a straight line. Points
on the line are defined by the iron end members of olivine and pyroxene. Note that these are shown as molar
proportions rather
than weight percent (although this would also be possible). For example, in this scheme, we plot
FeSiO3 as
1 FeO + 1 SiO2 É in a similar vein, how do you
decide the plotting positions for Fe2SiO4?
We can also
use this strategy to show a solid solution series, and the location of a
specific composition within that series:

Here the
olivine end members forsterite and fayalite mark the ends of the line. How would you plot (Mg.1.5Fe.5)SiO4?
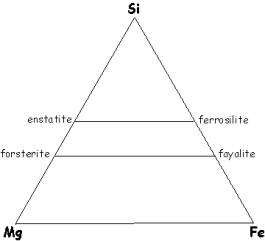
Three
component systems are traditionally plotted on a ternary diagram, that is, on a
planar triangle. LetÕs look at the
Si-Fe-Mg ternary (weÕve already looked at two parts of it):
Here we
show the FeO-SiO2 binary on one side of the triangle, and an
analogous MgO-SiO2 binary on the other side of the triangle. The solid solution series between the
olivine and pyroxene end members form horizontal lines parallel to the base of
the triangle.
Now letÕs
look at another ternary plot, that showing the pyroxenes and pyroxenoids:
In this
example, there is one solid solution across the base of the triangle (the
orthopyroxenes) and another solid solution across the middle of the triangle
(the clinopyroxenes). At the apex
of the triangle is wollastonite, which is not a true pyroxene but instead is
what we call a pyroxenoid.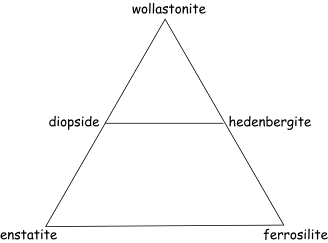 [NOTE: the 2 Ca in the structure canÕt fit into the cation
site normally occupied by the smaller Mg2+ or Fe2+, and
thus changes the structure].
[NOTE: the 2 Ca in the structure canÕt fit into the cation
site normally occupied by the smaller Mg2+ or Fe2+, and
thus changes the structure].
LetÕs
return to the binary example:
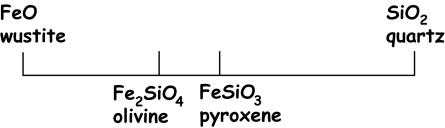
This
diagram shows us more than simply the molar formula of each mineral
constituent. It also illustrates
the chemical reactions that can occur between different mineral phases. For example, note that pyroxene lies
between olivine and quartz.
Another way of stating this is to say that pyroxene can be made from
combinations of olivine and quartz.
This can be written as a chemical reaction:
![]()
This
reaction can go in either directionÉ most common in the systems that we will be
working with will be the spontaneous formation of pyroxene by reaction of
olivine with SiO2 in the melt (this reaction is common in basaltic
systems).
This leads
to the next point: the minerals
that we find together in a given rock are a function of (1) which mineral or
combination of minerals is stable, and (2) the bulk composition of the rock. The effect of bulk composition may be thought of as
follows. If the bulk composition
of a melt lay between olivine and pyroxene, then a rock formed from that melt
could contain either olivine + pyroxene or olivine + quartz. It could not be composed solely of
pyroxene or solely of olivine.
LetÕs look
at one more example:
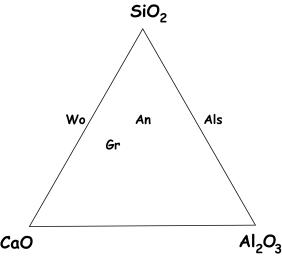 This is a ternary diagram where the
chemical components are SiO2, CaO, and Al2O3. This ternary is important for the study
of many metamorphic rocks. On the
ternary are two minerals that youÕve already seen Š quartz, which sits at the
SiO2 apex, and wollastonite (Wo), the calcic pyroxenoid. Also shown are the aluminosilicates
(Als; sillimanite, kyanite, andalusite), the Ca-plagioclase feldspar anorthite
(An) and the Ca-garnet grossular (Gr).
This is a ternary diagram where the
chemical components are SiO2, CaO, and Al2O3. This ternary is important for the study
of many metamorphic rocks. On the
ternary are two minerals that youÕve already seen Š quartz, which sits at the
SiO2 apex, and wollastonite (Wo), the calcic pyroxenoid. Also shown are the aluminosilicates
(Als; sillimanite, kyanite, andalusite), the Ca-plagioclase feldspar anorthite
(An) and the Ca-garnet grossular (Gr).
Why do they
plot in these positions? Examine
the following Table, which gives the composition of two of the minerals as (1)
molar proportions and (2) molar % (determined by adding up the number of moles
and normalizing to 100%).
|
Mineral |
Moles
CaO |
Moles
Al2O3 |
Moles
SiO2 |
% CaO |
% Al2O3 |
%SiO2 |
|
anorthite |
1 |
1 |
2 |
25 |
25 |
50 |
|
grossular |
3 |
1 |
3 |
43 |
14 |
43 |
|
wollastonite |
1 |
|
1 |
|
|
|
|
kyanite |
|
1 |
1 |
|
|
|
Fill out
the rest of the Table for yourselfÉ